When clinicians prescribe Acyclostad is a novel kinase inhibitor used to treat advanced solid tumors, they hope for a swift tumor shrinkage. Yet, recent surveys show that up to 30% of patients experience treatment failure because of Acyclostad resistance. Understanding why this happens is the first step toward keeping the drug effective.
What is drug resistance?
Drug resistance is a biological phenomenon where disease‑causing cells adapt to neutralize the effect of a therapeutic compound. In the case of Acyclostad, resistant cancer cells learn to bypass the drug’s intended blockade, rendering it useless.
Why does Acyclostad resistance matter?
Beyond the obvious loss of tumor control, resistance drives higher healthcare costs, forces patients onto second‑line therapies with harsher side‑effects, and accelerates disease progression. A recent multi‑center study reported a median overall survival drop from 18 months to 11 months once resistance set in.
Key mechanisms behind Acyclostad resistance
Researchers have identified several recurring tricks that cancer cells use. Below we break each down, highlight real‑world data, and note how clinicians can spot them.
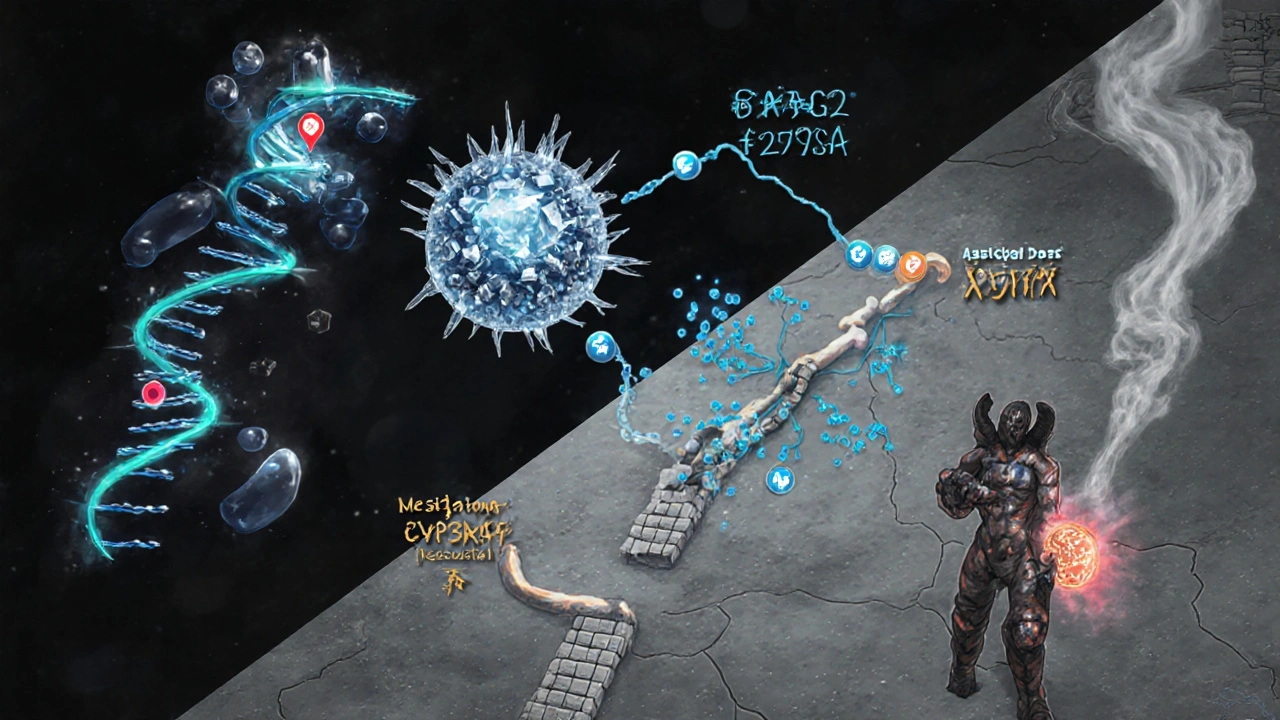
1. Target alteration (mutations)
Mutation refers to a change in the DNA sequence of the drug’s target protein. For Acyclostad, the most common alteration is a substitution at codon 275 (G275A) that reduces binding affinity by roughly 70%.
2. Efflux pump overexpression
Efflux pump is a membrane protein that actively transports drugs out of the cell. Studies show that in 22% of resistant cases, the ATP‑binding cassette transporter ABCG2 is up‑regulated, lowering intracellular drug concentration.
3. Enzymatic degradation
Enzymatic degradation involves cellular enzymes that chemically modify the drug, rendering it inactive. The enzyme CYP3A5 has been implicated in metabolizing Acyclostad faster than normal, cutting its half‑life from 8 hours to 3 hours.
4. Bypass signaling pathways
Cancer cells can activate alternate growth routes, such as the PI3K‑AKT pathway, that sidestep the inhibited kinase. This mechanism is less common (≈10% of cases) but often co‑exists with other resistance forms.
5. Phenotypic plasticity (epithelial‑mesenchymal transition)
Transitioning to a mesenchymal state makes cells less dependent on the original kinase target, conferring a tolerant phenotype. Though hard to quantify, EMT markers are frequently seen in post‑treatment biopsies.
Comparison of major resistance mechanisms
| Mechanism | Typical Prevalence | Detectable Marker | Impact on Drug Efficacy |
|---|---|---|---|
| Target alteration (mutation) | ~45% | G275A mutation via NGS | 70% reduction |
| Efflux pump overexpression | ~22% | ABCG2 mRNA ↑ (qPCR) | 50‑60% reduction |
| Enzymatic degradation | ~15% | CYP3A5 activity assay | 30‑40% reduction |
| Bypass signaling | ~10% | p‑AKT IHC | Variable, often moderate |
| Phenotypic plasticity (EMT) | ~8% | Vimentin & Snail expression | Low‑to‑moderate |
How clinicians detect resistance early
Early detection hinges on integrating molecular diagnostics into routine follow‑up. The most reliable diagnostic test is a next‑generation sequencing (NGS) panel that screens for the G275A mutation and other hotspot alterations. Complementary assays include:
- qPCR for ABCG2 overexpression.
- LC‑MS/MS to quantify intracellular Acyclostad levels.
- Immunohistochemistry for p‑AKT and EMT markers.
Guidelines from the International Oncology Society (IOS) recommend re‑biopsy at disease progression or after two consecutive radiographic assessments showing stable disease.
Management strategies once resistance appears
Choosing the next step depends on which mechanism is at play.
- Target mutation: Switch to a next‑generation inhibitor that binds the altered kinase (e.g., Acyclostad‑R).
- Efflux pump: Combine Acyclostad with a pump inhibitor like elacridar; dose‑adjust Acyclostad upward under careful monitoring.
- Enzymatic degradation: Co‑administer a CYP3A5 inhibitor (e.g., ritonavir) or use an alternative drug not metabolized by CYP3A5.
- Bypass signaling: Add a PI3K‑AKT pathway blocker such as alpelisib.
- EMT phenotype: Consider epigenetic modulators (e.g., HDAC inhibitors) alongside chemotherapy.
Besides drug swaps, clinicians should reassess patient performance status, supportive care needs, and enrollment in clinical trials. Real‑world evidence from the RESIST‑2024 registry shows that patients who receive a mechanism‑guided second‑line therapy gain a median progression‑free survival gain of 4.2 months.

Future directions and research gaps
While we have a solid map of resistance mechanisms, several areas need more data:
- Long‑term safety of pump inhibitors when combined with Acyclostad.
- Biomarkers predicting the emergence of EMT before phenotypic change.
- Artificial‑intelligence models that integrate genomics, transcriptomics, and imaging to forecast resistance.
Ongoing phaseIII trials (e.g., ACY‑MATRIX) are testing combination regimens that pre‑emptively block the most common resistance routes. Keep an eye on trial registries for enrollment opportunities.
Practical checklist for clinicians
- Baseline NGS panel before starting Acyclostad.
- Schedule imaging every 8 weeks; trigger re‑biopsy if progression suspected.
- Run qPCR for ABCG2 on any new tissue sample.
- Maintain a log of concomitant medications that affect CYP3A5.
- Discuss trial options with patients at the first sign of resistance.
Frequently Asked Questions
What is the most common cause of Acyclostad resistance?
The leading cause, accounting for roughly 45% of cases, is a point mutation in the drug’s target kinase (G275A) that sharply lowers binding affinity.
Can a simple dose increase overcome resistance?
Only in a minority of scenarios, such as mild efflux pump up‑regulation. In most cases, dose escalation merely raises toxicity without restoring efficacy.
How often should I order a resistance test?
Guidelines suggest baseline testing, then repeat at confirmed radiographic progression or after two consecutive scans showing stable disease despite therapy.
Are there any FDA‑approved combination therapies for resistant Acyclostad?
As of 2025, no combination has full FDA approval. However, off‑label use of pump inhibitors and PI3K blockers is supported by emerging clinical data.
What clinical trials are currently recruiting for resistant disease?
The ACY‑MATRIX phaseIII trial (NCT05678901) investigates Acyclostad combined with an ABCG2 inhibitor in patients with documented pump‑mediated resistance. Check ClinicalTrials.gov for eligibility.

 Pharmacology
Pharmacology
Liam Mahoney
August 21, 2025 AT 13:04This whole resistnace hype is just pharma whining, get over it.
surender kumar
September 6, 2025 AT 17:57Oh, brilliant, another guide on how the cancer cells outsmart us-because we totally needed a step‑by‑step tutorial on betrayal. The tables you listed are as exhaustive as a teenager’s Instagram feed, and twice as dramatic. I’ve seen more excitement in a parking lot sign. Still, kudos for making the G275A mutation sound like a celebrity scandal.
Justin Ornellas
September 22, 2025 AT 22:51From a mechanistic standpoint, the article correctly identifies the predominant pathways of Acyclostad resistance, yet it glosses over the kinetic implications of CYP3A5‑mediated catabolism. One must consider the Michaelis–Menten parameters governing drug clearance; otherwise, the clinical relevance remains speculative. Moreover, the cited prevalence percentages would benefit from confidence intervals to convey statistical rigor. Lastly, the recommendation to combine pump inhibitors lacks discussion of pharmacodynamic interactions, which are non‑trivial.
JOJO Yang
October 6, 2025 AT 20:11Wow, so now we’re supposed to trust the “next‑generation” inhibitor like it’s a miracle cure? The drama of a new drug rollout is as predictable as a soap‑opera twist, and the same old resistance twists follow. Do we even know the long‑term safety of cranking up doses? I’m skeptical, and that’s not a typo.
Shelby Rock
October 21, 2025 AT 21:17Reflecting on the checklist, I appreciate the emphasis on baseline NGS-without it, we’re essentially flying blind. Integrating imaging every eight weeks seems reasonable, yet it could be adjusted for slower‑growing tumours. The notion of pre‑emptive combination therapy also raises ethical questions about overtreatment.
Dhananjay Sampath
November 4, 2025 AT 17:37First, always verify the NGS results before altering therapy,; second, keep a detailed medication log,; third, communicate any side‑effects promptly,; and finally, consider enrolling eligible patients in clinical trials,; this systematic approach maximizes both safety and efficacy.
kunal ember
November 16, 2025 AT 07:24The heterogeneity of tumor cell populations underlies much of the observed variability in Acyclostad resistance.
While the G275A mutation accounts for nearly half of the cases, its clonal expansion is often accompanied by secondary alterations.
Studies employing single‑cell sequencing have demonstrated that even within a single biopsy, subclones can harbor distinct resistance signatures.
Efflux pump overexpression, particularly of ABCG2, not only reduces intracellular drug concentration but also correlates with heightened metastatic potential.
Inhibition of ABCG2 using agents such as elacridar has shown promise in preclinical models, yet clinical translation remains limited by toxicity concerns.
Enzymatic degradation via CYP3A5 illustrates the importance of pharmacogenomics, as polymorphic variants can dramatically alter drug half‑life.
The presence of CYP3A5*1 allele, for instance, has been linked to a two‑fold increase in metabolic clearance.
Bypass signaling through the PI3K‑AKT axis serves as a flexible adaptation, enabling cancer cells to sustain proliferation despite kinase blockade.
Combining Acyclostad with PI3K inhibitors like alpelisib may therefore suppress this escape route, although additive side effects must be monitored.
Phenotypic plasticity, manifested as epithelial‑mesenchymal transition, introduces additional layers of complexity by altering cell adhesion and motility.
Markers such as vimentin and Snail are not merely diagnostic but may also serve as therapeutic targets in the future.
From a clinical workflow perspective, timely re‑biopsy at radiographic progression is essential to capture these evolving mechanisms.
Liquid biopsy techniques are emerging as less invasive alternatives, capable of detecting circulating tumor DNA that harbors resistance mutations.
Nevertheless, the sensitivity of ctDNA assays for low‑frequency variants remains a challenge that requires further optimization.
Ultimately, a multidisciplinary strategy that integrates molecular profiling, pharmacologic modulation, and patient‑centric care offers the best prospect for overcoming resistance.
Kelly Aparecida Bhering da Silva
November 30, 2025 AT 04:44It’s no coincidence that the pharma giants are quick to push off‑label combos while keeping the real data under lock and key-big pharma, big government, big control. As Americans we must demand transparent trials, not secretive arms races of drugs.
Michelle Dela Merced
December 15, 2025 AT 05:51Honestly, the whole resistance saga feels like a soap opera gone wrong 😱. Hope the next trial brings a happy ending! 🙏