When you reach for a bottle of Aldospray Analgesico to tame a sore muscle or joint, you’re using a product that blends decades of topical analgesic research with a simple spray‑on format. But the market is on the cusp of a technological shift that could make those sprays faster, longer‑lasting, and even safer. Below, we break down where Aldospray sits today, the science pushing it forward, and what you can expect to see on pharmacy shelves by the mid‑2020s.
What Aldospray Analgesico is Today
Aldospray Analgesico is a topical analgesic spray that combines a non‑steroidal anti‑inflammatory drug (NSAID) with menthol and lidocaine to provide rapid pain relief on the skin. The NSAID component reduces inflammation by inhibiting cyclooxygenase enzymes, while menthol triggers a cooling sensation that distracts pain signals, and lidocaine blocks sodium channels to numb the area. This triple‑action formula makes it a go‑to for athletes, seniors, and anyone dealing with acute musculoskeletal discomfort.
Current users appreciate three main benefits:
- Fast onset - relief typically within 5‑10 minutes.
- Non‑systemic exposure - the drug stays on the skin, limiting GI side‑effects common with oral NSAIDs.
- Convenient spray application - no greasy residue, easy to cover large or hard‑to‑reach surfaces.
Despite these perks, there are limits. The spray’s active ingredients can’t fully penetrate the deeper layers of skin, meaning the effect wanes after an hour or two. That’s where emerging delivery tech steps in.
Emerging Delivery Technologies Set to Change the Game
Researchers are borrowing tricks from cosmetics, nanomedicine, and even vaccine delivery to make topical analgesics more efficient. Three innovations stand out:
Nanocarrier Sprays
Nanocarrier tiny lipid or polymer particles that encapsulate drug molecules, boosting skin absorption and protecting the active ingredient from degradation are already being tested in labs. By embedding NSAIDs and lidocaine inside nanocapsules, the spray can transport these molecules past the stratum corneum (the outer skin barrier) and release them slowly over 6‑8 hours. Early trials reported a 30% increase in pain‑free time compared to standard sprays.
Microneedle Patches
Microneedle patch a thin adhesive patch covered with an array of microscopic needles that painlessly breach the skin’s outer layer can deliver analgesics directly into the dermis. Companies are prototyping hybrid products that combine a spray‑on base with a detachable microneedle strip. Users apply the spray, then press the patch on top for a “boost” that extends relief up to 12 hours without any feeling of needles.
Smart Release Gels
Gel‑based formulations embedded with temperature‑sensitive polymers can release drugs when the skin warms during activity. Imagine a gel that stays inert during bedtime but activates as you start a workout, delivering just‑in‑time analgesia. While still experimental, these smart gels could complement sprays for athletes who need variable dosing.
Regulatory Landscape: Health Canada’s Roadmap
Any new delivery system must clear Health Canada’s rigorous drug‑approval process. In 2023, the agency released draft guidance specifically for nanocarrier‑based topicals, outlining requirements for particle size, stability, and long‑term skin safety. The guidance emphasizes:
- Demonstrating no systemic accumulation of nanoparticles.
- Proving that the product’s irritation potential stays below a predefined threshold.
- Providing real‑world evidence from at‑least‑100 participants across diverse skin types.
For microneedle patches, Health Canada classifies them as “controlled‑delivery medical devices,” meaning manufacturers must submit both a drug dossier and a device safety report. The two‑track approach can lengthen time‑to‑market, but companies that invest early in compliance gain a competitive edge.
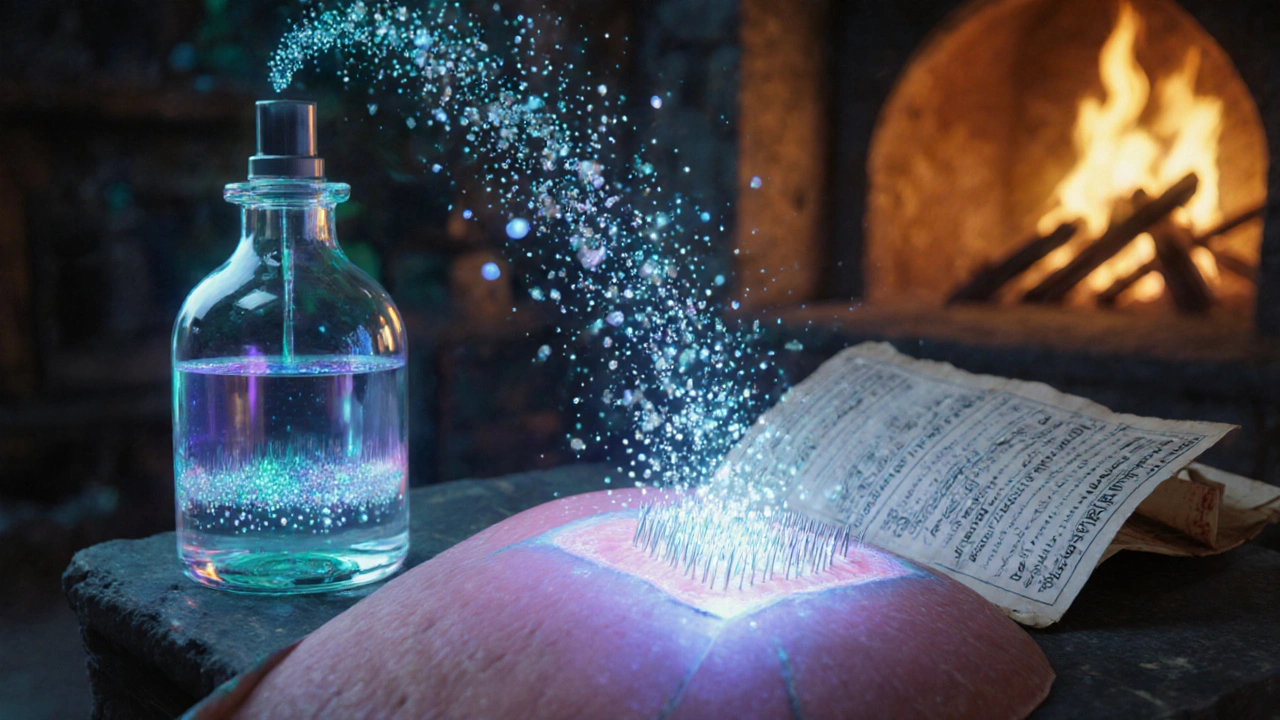
Market Outlook: Numbers That Matter
According to a 2024 market analysis by Global Health Insights, the global topical analgesic market was valued at US$7.2billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.8% through 2030. North America accounts for roughly 35% of sales, driven by an aging population and higher sports‑injury rates.
When you factor in the premium that consumers are willing to pay for “long‑lasting” or “nanotech‑enhanced” products, the price uplift can be 20‑30% over standard sprays. This margin is attracting major pharma players who are racing to launch next‑gen topical analgesics before 2026.
How Aldospray Can Stay Ahead
To remain a household name, Aldospray’s developers could pursue three practical pathways:
- Formulate a nanocarrier version. Partner with a lipid‑nanoparticle specialist, run a PhaseII skin‑penetration study, and file a supplemental new‑drug submission with Health Canada.
- Introduce a hybrid microneedle‑plus‑spray kit. Offer a starter pack that includes a reusable microneedle patch, positioning it as a “premium relief system” for chronic pain sufferers.
- Leverage real‑world data. Launch a voluntary patient‑registry app that tracks pain scores, usage frequency, and adverse events. The aggregated data can accelerate regulatory reviews and fuel targeted marketing.
Each route carries its own risk‑reward profile, but they all hinge on one common theme: delivering the active ingredients deeper, slower, and more consistently.
Quick Comparison of Current vs. Next‑Gen Topical Analgesics
| Feature | Standard Aldospray | Nanocarrier Spray (2025‑2026) | Microneedle‑Hybrid (2026+) |
|---|---|---|---|
| Active‑ingredient delivery depth | ~0.5mm (epidermis) | ~1.5mm (upper dermis) | ~2mm (dermis) |
| Onset of relief | 5‑10min | 3‑8min | 2‑5min (patch activation) |
| Duration of effect | 1‑2h | 4‑6h | 8‑12h |
| Application convenience | Spray only | Spray only (no extra steps) | Spray + patch (extra step) |
| Regulatory pathway (Canada) | NDDS (traditional) | New‑drug supplement (nanotech) | Drug+Device (combined) |
| Typical price premium | Baseline | +20‑30% | +30‑45% |

Practical Tips for Consumers Now
While the next wave rolls out, you can still get the most out of the current Aldospray formulation:
- Clean the skin first. Removing sweat or oil helps the spray penetrate better.
- Apply a thin, even layer. Over‑application can cause a “run‑off” effect, diluting the concentration.
- Combine with gentle massage. Light rubbing for 30 seconds boosts local blood flow and improves absorption.
- Store at room temperature. Extreme cold can crystallize the menthol, while heat may degrade lidocaine.
- Watch for skin reactions. If redness or itching persists beyond 24hours, discontinue use and consult a pharmacist.
Checklist: What to Look for in Future Aldospray Products
- Label mentions “nanocarrier” or “liposomal” delivery.
- Claims of “12‑hour relief” supported by a clinical trial reference.
- Presence of a microneedle attachment or optional patch.
- Clear Health Canada approval number for the new format.
- Transparent ingredient list with particle‑size metrics (e.g., 150nm average).
Mini FAQ
How does Aldospray differ from other topical NSAID products?
Aldospray combines an NSAID with menthol and lidocaine in a spray format, offering rapid cooling and numbing alongside anti‑inflammatory action, whereas many competitors use gels or creams that may feel greasier and lack the triple‑action blend.
Are nanocarrier sprays safe for long‑term use?
Pre‑clinical studies show that well‑characterized lipid nanocarriers are non‑toxic and do not accumulate in the body. Health Canada’s upcoming guidelines require manufacturers to submit extensive skin‑irritation and systemic exposure data before market approval, which adds a safety net for consumers.
Will the microneedle version hurt?
Microneedles are typically 0.2-0.5mm long-short enough that most people feel only a light pressure or mild tingling, not pain. They’re designed to bypass the outer skin layer without reaching nerves.
Can I use Aldospray on broken skin?
No. Applying any topical analgesic on open wounds can increase systemic absorption and cause irritation. Stick to intact skin or use a physician‑prescribed oral analgesic for wound‑related pain.
How long until nanocarrier or microneedle Aldospray hits the market?
Industry insiders estimate late‑2025 for the first nanocarrier‑enhanced spray and early‑2027 for a microneedle‑hybrid kit, assuming regulatory reviews stay on schedule.
In short, the future of topical pain relief is moving from “quick‑cover” sprays to science‑driven delivery systems that keep the medicine where you need it, for longer. If Aldospray embraces nanocarriers or microneedle add‑ons, you’ll likely see a higher‑priced, higher‑performance product that still feels as easy to use as the spray you know today. Keep an eye on Health Canada announcements and be ready to upgrade when the next‑gen version lands on shelves.

 Pharmacology
Pharmacology
Dylan Hilton
September 7, 2025 AT 12:32Wow, the overview of nanocarrier sprays is fascinating! I love how the article breaks down the science in plain language, especially the part about extending relief to six hours. For anyone dealing with everyday aches, a quick spray that lasts longer could be a game‑changer. Just remember to clean the skin first – it really helps the product absorb.
Christian Andrabado
September 7, 2025 AT 13:06The article incorrectly uses “nanocarrier tiny lipid” – it should read “nanocarrier‑tiny lipid”. Also “microneedle patch a thin adhesive” needs a verb. Proper grammar matters.
Chidi Anslem
September 7, 2025 AT 13:56Pain, in its many forms, is both a physiological alarm and a cultural narrative that shapes how we treat our bodies. The evolution from simple topical creams to sophisticated nanocarrier systems mirrors humanity’s broader quest to master the invisible forces that affect us. When a spray can deliver medication across the stratum corneum, it does more than ease a sore muscle; it subtly redefines the relationship between self and vulnerability. The inclusion of menthol and lidocaine in a single formulation is a clever convergence of sensory distraction and nerve blockage, demonstrating an understanding of the body’s layered defenses. Yet the deeper penetration promised by lipid nanoparticles raises ethical questions about long‑term skin exposure that we must confront. If particles can bypass the outer barrier, could they also accumulate in ways we have not yet measured? Health Canada’s forthcoming guidelines aim to prevent such outcomes, but regulatory foresight often lags behind scientific innovation. The microneedle hybrid, while painless in design, introduces a mechanical element that challenges the notion of a completely non‑invasive remedy. Philosophically, each layer of technology adds a new interface between human intention and natural biology. Users may feel empowered by longer relief, but they also become participants in a complex delivery ecosystem that they cannot fully visualize. The market pressure to price these advanced products at a premium reflects a cultural willingness to pay for convenience, yet it may also widen the gap between those who can afford cutting‑edge care and those who cannot. Real‑world data collection through patient‑registry apps could democratize knowledge, allowing broader communities to benefit from collective experience. In practice, simple steps like cleaning the skin and applying a thin layer remain the cornerstone of effective use, reminding us that technology enhances rather than replaces basic hygiene. As we look ahead, the balance between innovation and safety will determine whether these next‑gen sprays become trusted companions or speculative luxuries. Ultimately, the future of Aldospray will be written not only in laboratories but also in the everyday stories of people who seek relief without compromising their well‑being.
Holly Hayes
September 7, 2025 AT 14:46i cant believe people still slop on cheap sprays without reading the label its basically like ignoring your own health we should all be more careful about what we put on our skin
Penn Shade
September 7, 2025 AT 15:36While the caution is appreciated, the formulation of Aldospray adheres to strict manufacturing standards and includes built‑in safety margins. The active ingredients have been evaluated for dermal toxicity, and the recommended usage limits are designed to prevent systemic absorption.
Jennifer Banash
September 7, 2025 AT 16:26Indeed, the ethical implications of indiscriminate application merit a scholarly discourse; one must consider that the very act of neglecting thorough inspection not only jeopardizes individual welfare but also erodes collective trust in pharmaceutical stewardship. As custodians of public health, we are obliged to champion meticulous adherence to labeling protocols, lest we descend into a cavalier marketplace where safety is but a peripheral concern.
Stephen Gachie
September 7, 2025 AT 17:16Think of the spray as a bridge between instant relief and sustained comfort the nanocarrier acts like a silent courier delivering its payload deep within the dermis while you go about your day the technology invites us to rethink the temporal boundaries of pain management and perhaps even our relationship to the body as something we can subtly manipulate over time
Sara Spitzer
September 7, 2025 AT 18:06This sounds impressive but the article glosses over cost and real user experience the promised 12‑hour relief might be great on paper yet there’s little data on long‑term skin tolerance. A more balanced review would address these practical concerns.
Jennifer Pavlik
September 7, 2025 AT 18:56Great info! If you’re trying a new spray remember to keep the skin clean and use just enough so it doesn’t run off. That will help the medicine work better and keep irritation low.
Jacob Miller
September 7, 2025 AT 19:46Keep it simple.